Biochemistry and regulation of metabolism in acetogenic bacteria
1. Lactate metabolism
Lactate is a common substrate for bacteria, under aerobic as well as anaerobic conditions. However, anaerobes are faced with an enormous problem. The usual electron acceptor NAD (E0’ = -320 mV) cannot be used, since the redox potential of the lactate/pyruvate pair is to electropositive (E0’ = -190 mV). The solution to this long-standing problem in anaerobic biochemistry was presented recently. A. woodii has a novel type of lactate dehydrogenase that forms a complex with an electron-transfer flavoprotein (Etf). This complex oxidizes lactate with concomitant reduction of NAD. The energy required for the uphill transport of electrons to NAD is provided by the simultaneous oxidation of reduced ferredoxin:
lactate + Fd2- + 2 NAD+ -> pyruvate + Fd + 2 NADH
by electron confurcation, a reversal of electron bifurcation. Lactate metabolism is depicted in the figure below. It is a prime example of a metabolism in which the Rnf complex works backwards: it drives ferredoxin reduction at the expense of a chemiosmotic ion potential established by a reversal of the ATP synthase reaction. ATP is only synthesized by substrate-level-phosphorylation.
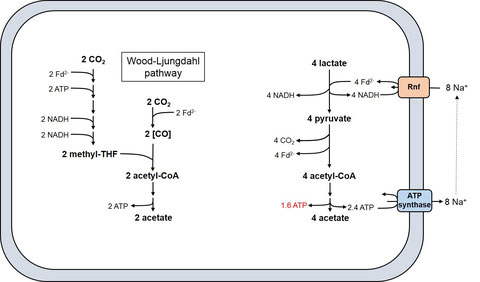
Lactate metabolism in Acetobacterium woodii. Fd; ferredoxin
References
Weghoff, M.C., Bertsch, J., Müller, V. (2014) A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol., doi:10.1111/1462-2920.12493.
Highlight in:
Schink, B. (2014) Electron confurcation in anaerobic lactate oxidation. Environ. Microbiol., in press.

